Chemistry College answer answered Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with 4.000 moles of o2. 2 c8h18 (l) + 25 o2 (g) → 16 co2 (g) + 18 h2o (g) molar mass co2 = 44.01 g/mol Advertisement liyahlove5358 is waiting for your help. Add your answer and earn points. plus Add answer +5 pts
Solved Give the percent yield when 28.16 g of CO2 are formed | Chegg.com
Expert Answer. 100% (4 ratings) Transcribed image text: PartA Give the percent yield when 28.16 g of CO2 are formed from the reaction of 14.00 moles of C8 His with 8.000 moles of O2 2 C8H18 + 2502 ? 16 CO2 + 18H20 28.56% 1 1.42% 7.140% 14.28% 12.50%. Previous question Next question.

Source Image: yumpu.com
Download Image
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 4.000 moles of O2. 2C8H18 + 24O2→16CO2 + 18H2O Introduction to General, Organic and Biochemistry 11th Edition ISBN: 9781285869759 Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres

Source Image: numerade.com
Download Image
Solved Give the percent yield when 28.16 g of CO2 are formed | Chegg.com
Question. Transcribed Image Text: Question 1 Give the percent yield when 28.16 g of CO 2 are formed from the reaction of 8.000 moles of C gH 18 with 4.000 moles of O 2. 2 C8H18+ 25 02 → 16 CO2 + 18 H20 20.00% O 25.00% O 50.00% O 12.50% A Moving to another question will save this response. MacBook Pro @ $ 7 8. W E R T Y S K C V B N M command # 3.

Source Image: brainly.com
Download Image
Give The Percent Yield When 28.16 G Of Co2
Question. Transcribed Image Text: Question 1 Give the percent yield when 28.16 g of CO 2 are formed from the reaction of 8.000 moles of C gH 18 with 4.000 moles of O 2. 2 C8H18+ 25 02 → 16 CO2 + 18 H20 20.00% O 25.00% O 50.00% O 12.50% A Moving to another question will save this response. MacBook Pro @ $ 7 8. W E R T Y S K C V B N M command # 3.
Nov 28, 2023To calculate the percent yield, we convert 28.16 g of CO2 formed into moles, determine the limiting reagent, calculate the theoretical yield of CO2 in grams based on the moles of limiting reagent, and then divide the actual yield by the theoretical yield and multiply by 100. Explanation:
How many moles of O2 are needed to produce 50.0 moles of CO2? 2 C8H18 + 25 O2 –> 16 CO2 + 18 H20 a) – brainly.com
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 8.000 moles of C8H18 with 4.000 moles of O2. 2C8H18+ 25 02 16 CO2 + 18 H₂O O 20.00% O 25.00% O 50.00% 12.50%. There are 2 steps to solve this one.
SOLUTION: CHEM 1103 Ch 1-3 Chemistry Practice Exam – Studypool
Source Image: studypool.com
Download Image
Answered: Give the percent yield when 28.16 g of… | bartleby
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 8.000 moles of C8H18 with 4.000 moles of O2. 2C8H18+ 25 02 16 CO2 + 18 H₂O O 20.00% O 25.00% O 50.00% 12.50%. There are 2 steps to solve this one.
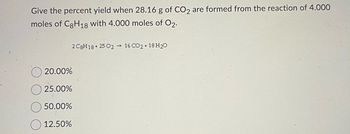
Source Image: bartleby.com
Download Image
Solved Give the percent yield when 28.16 g of CO2 are formed | Chegg.com
Chemistry College answer answered Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with 4.000 moles of o2. 2 c8h18 (l) + 25 o2 (g) → 16 co2 (g) + 18 h2o (g) molar mass co2 = 44.01 g/mol Advertisement liyahlove5358 is waiting for your help. Add your answer and earn points. plus Add answer +5 pts

Source Image: chegg.com
Download Image
Solved Give the percent yield when 28.16 g of CO2 are formed | Chegg.com
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 4.000 moles of O2. 2C8H18 + 24O2→16CO2 + 18H2O Introduction to General, Organic and Biochemistry 11th Edition ISBN: 9781285869759 Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
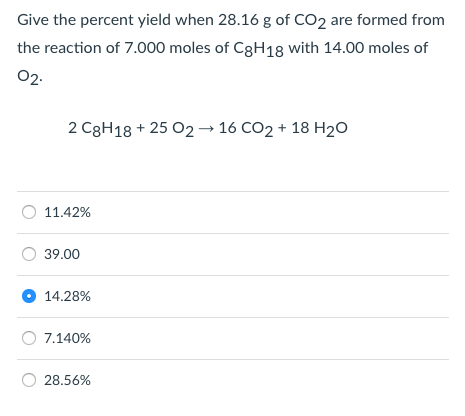
Source Image: chegg.com
Download Image
Answered: Give the percent yield when 28.16 g of… | bartleby
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 2.000 moles of C8H18 with 4.000 moles of O2.2 C8H18 + 25 O2 → 16 CO2 + 18 H2O Chemistry & Chemical Reactivity 9th Edition ISBN: 9781133949640 Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
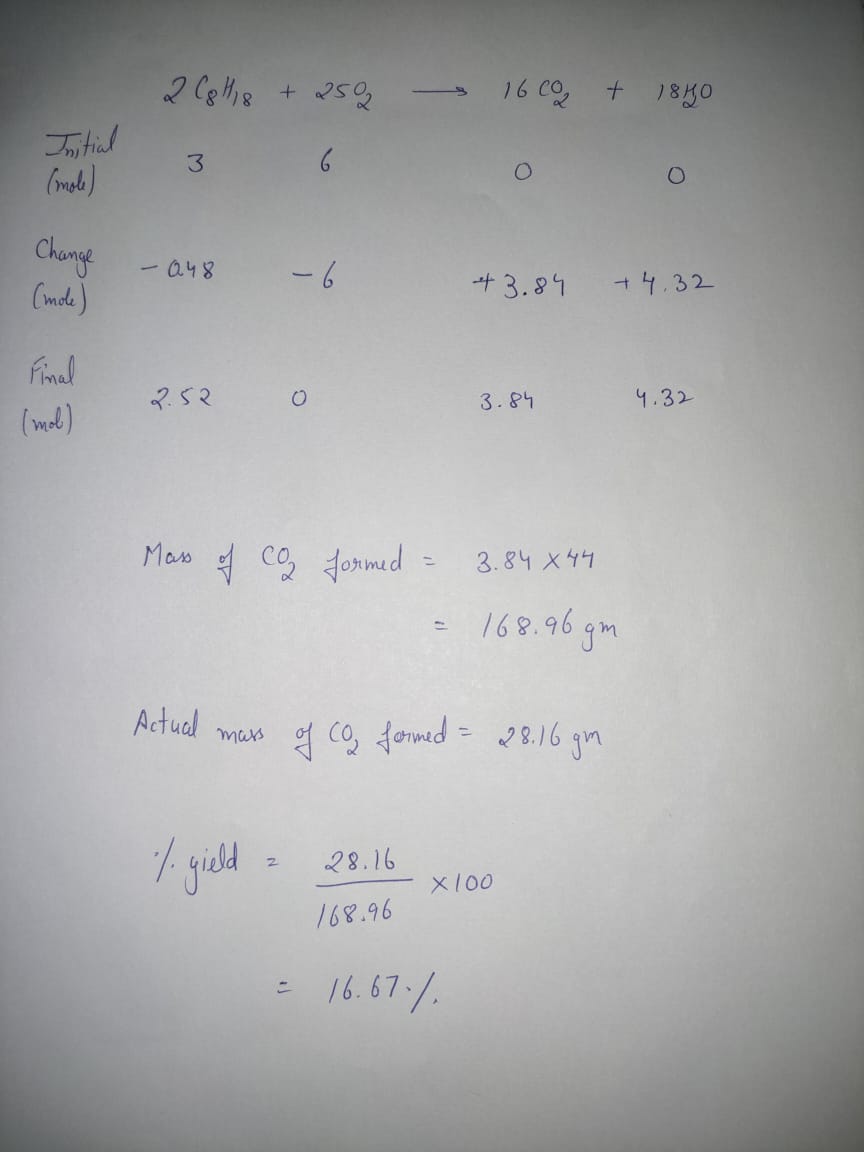
Source Image: bartleby.com
Download Image
SOLUTION: CHEM 1103 Ch 1-3 Chemistry Practice Exam – Studypool
Question. Transcribed Image Text: Question 1 Give the percent yield when 28.16 g of CO 2 are formed from the reaction of 8.000 moles of C gH 18 with 4.000 moles of O 2. 2 C8H18+ 25 02 → 16 CO2 + 18 H20 20.00% O 25.00% O 50.00% O 12.50% A Moving to another question will save this response. MacBook Pro @ $ 7 8. W E R T Y S K C V B N M command # 3.
Source Image: studypool.com
Download Image
Untitled
Nov 28, 2023To calculate the percent yield, we convert 28.16 g of CO2 formed into moles, determine the limiting reagent, calculate the theoretical yield of CO2 in grams based on the moles of limiting reagent, and then divide the actual yield by the theoretical yield and multiply by 100. Explanation:
Source Image: turkser.org.tr
Download Image
Answered: Give the percent yield when 28.16 g of… | bartleby
Untitled
Expert Answer. 100% (4 ratings) Transcribed image text: PartA Give the percent yield when 28.16 g of CO2 are formed from the reaction of 14.00 moles of C8 His with 8.000 moles of O2 2 C8H18 + 2502 ? 16 CO2 + 18H20 28.56% 1 1.42% 7.140% 14.28% 12.50%. Previous question Next question.
Solved Give the percent yield when 28.16 g of CO2 are formed | Chegg.com SOLUTION: CHEM 1103 Ch 1-3 Chemistry Practice Exam – Studypool
Give the percent yield when 28.16 g of CO2 are formed from the reaction of 2.000 moles of C8H18 with 4.000 moles of O2.2 C8H18 + 25 O2 → 16 CO2 + 18 H2O Chemistry & Chemical Reactivity 9th Edition ISBN: 9781133949640 Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel